The Role of 2-Bromoethanol in Synthesis: A Comprehensive Guide
2-Bromoethanol plays a crucial role in organic synthesis, especially in chemical and pharmaceutical industries. This compound, known for its reactivity, is widely used as an intermediate in the production of various chemicals.
2-Bromoethanol plays a crucial role in organic synthesis, especially in chemical and pharmaceutical industries. This compound, known for its reactivity, is widely used as an intermediate in the production of various chemicals. Understanding the uses, properties, and handling of 2-Bromoethanol is essential for any professional working with synthetic compounds. This comprehensive guide will explore its importance, how it is used in synthesis, and what precautions need to be taken when handling it.
What is 2-Bromoethanol?
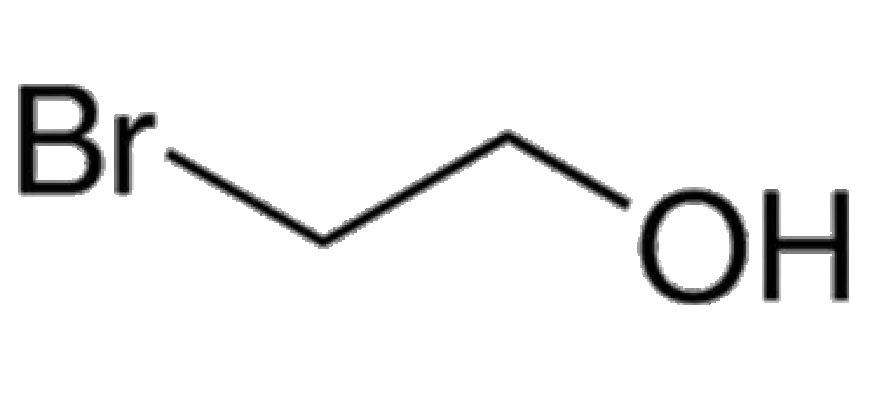
2-Bromoethanol is an organic compound with the chemical formula C₂H₅BrO. It is a clear, colorless liquid with a faint, pleasant odor. The compound is known for its ability to act as an alkylating agent, making it highly valuable in the synthesis of complex molecules. In synthetic organic chemistry, 2-Bromoethanol is used to introduce hydroxyethyl groups into a wide range of compounds, offering flexibility in the design and construction of chemical frameworks.
Chemical Properties of 2-Bromoethanol
2-Bromoethanol exhibits several important chemical properties that make it a versatile reagent in organic synthesis:
- Molecular Weight: 124.98 g/mol
- Boiling Point: 148°C
- Density: 1.684 g/mL at 25°C
- Reactivity: It is highly reactive due to the presence of both bromine and hydroxyl functional groups. The bromine atom serves as a good leaving group, facilitating substitution reactions.
These properties allow 2-Bromoethanol to engage in numerous chemical reactions including nucleophilic substitution, alkylation, and more, making it an essential tool in the synthesis of complex organic molecules.
Uses of 2-Bromoethanol in Synthesis
The primary application of 2-Bromoethanol in chemical synthesis lies in its ability to act as an alkylating agent. This function allows it to introduce an ethyl group into various organic molecules, which is essential for building more complex structures.
1. Etherification Reactions
In organic synthesis, 2-Bromoethanol is widely used in etherification reactions. The hydroxyl group (-OH) in 2-Bromoethanol makes it a suitable precursor for creating ethers, especially in reactions where selective alkylation is required. The use of 2-Bromoethanol facilitates the introduction of a hydroxyethyl group, which is key in many pharmaceutical intermediates.
2. Synthesis of Surfactants and Emulsifiers
2-Bromoethanol is used in the production of surfactants and emulsifiers. Its reactivity with fatty acids leads to the formation of products that help lower surface tension between two immiscible liquids. This makes it a valuable component in the manufacturing of personal care products, detergents, and industrial emulsifiers.
3. Intermediate in Pharmaceuticals
In the pharmaceutical industry, 2-Bromoethanol is a critical intermediate in the synthesis of various bioactive molecules. Its reactivity is harnessed to modify active pharmaceutical ingredients (APIs), facilitating the production of drugs with enhanced efficacy. It is often involved in the synthesis of anti-cancer agents, antibiotics, and antifungal compounds.
4. Alkylation of Amines and Thiols
The bromine atom in 2-Bromoethanol makes it a valuable reagent for the alkylation of amines and thiols. These reactions are crucial in the preparation of quaternary ammonium salts and thioethers, both of which have numerous applications in chemical synthesis and industrial chemistry.
Safety Precautions When Handling 2-Bromoethanol
2-Bromoethanol is a hazardous chemical and should be handled with care. Prolonged exposure can result in adverse health effects, including skin irritation, respiratory distress, and potential organ toxicity. It is important to follow the safety guidelines when working with this compound.
1. Personal Protective Equipment (PPE)
When handling 2-Bromoethanol, the use of appropriate PPE is essential. This includes:
- Gloves: Nitrile or latex gloves to protect against skin contact.
- Eye Protection: Safety goggles or a face shield to prevent eye exposure.
- Lab Coat: To protect clothing and skin.
- Ventilation: Working in a fume hood is recommended to avoid inhalation of vapors.
2. Proper Storage
2-Bromoethanol should be stored in a cool, dry place, away from direct sunlight and sources of ignition. It is important to keep it tightly sealed in a well-ventilated area to prevent the release of hazardous fumes.
3. Disposal Procedures
Disposal of 2-Bromoethanol must follow local environmental regulations. It is classified as hazardous waste and should not be released into the environment. Working with a qualified waste disposal service ensures proper and safe disposal.
Environmental Impact of 2-Bromoethanol
2-Bromoethanol is considered a toxic compound and can have a detrimental effect on the environment if not handled correctly. It is important to minimize its release into water systems as it is harmful to aquatic life. Researchers and manufacturers using 2-Bromoethanol are encouraged to adopt green chemistry practices, aiming to reduce the overall environmental footprint by employing safer alternatives where possible.
Alternatives to 2-Bromoethanol
With growing concerns about the safety and environmental impact of hazardous chemicals, alternative reagents are being explored in synthetic chemistry. Some greener substitutes for 2-Bromoethanol include:
- Bio-based alkylating agents: Derived from renewable sources.
- Safer halogen-free alkylating reagents: Offering similar reactivity without the associated hazards.
However, despite these alternatives, 2-Bromoethanol continues to play a vital role due to its unique chemical properties and versatility in synthesis.
Conclusion
2-Bromoethanol is a powerful reagent in the field of synthetic chemistry, offering a wide range of applications in etherification, surfactant production, and pharmaceutical synthesis. While it must be handled with caution due to its hazardous nature, the benefits it provides in creating complex molecules are undeniable. By adhering to safety guidelines and adopting greener practices, researchers can continue to leverage 2-Bromoethanol's reactivity for innovative and sustainable chemical solutions. As a leading Tertiary Butyl Carbazate manufacturers in india, we prioritize quality and consistency in our production processes.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0