Why Outsourcing Medical and Scientific Affairs Services Improves Clinical Trial Outcomes
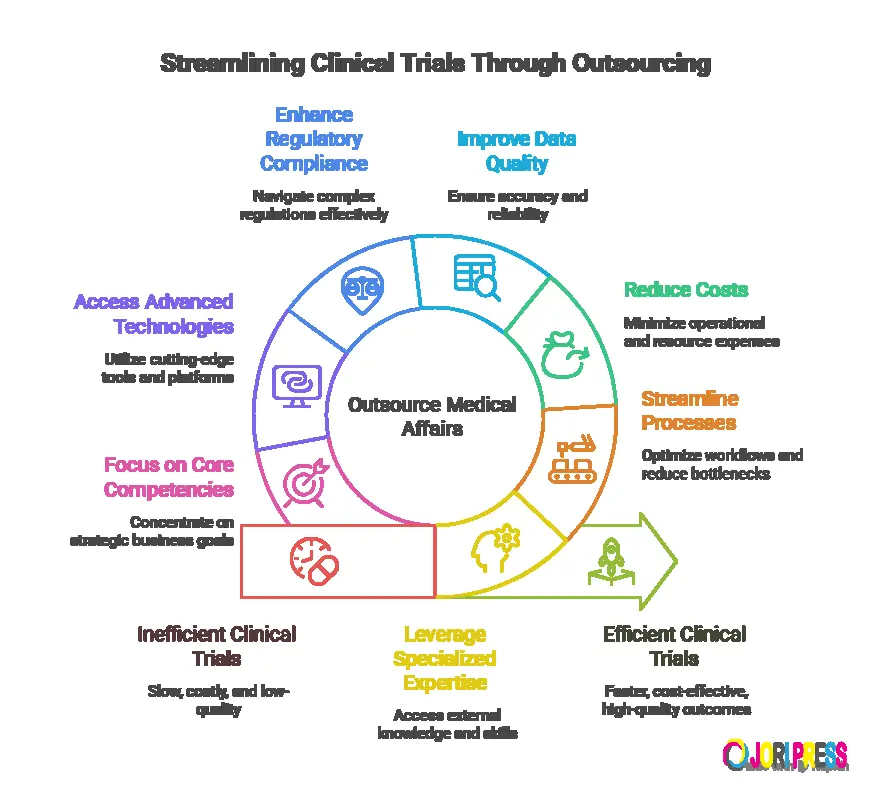
In today’s highly regulated and data-driven clinical research environment, sponsors and biotech companies are under constant pressure to generate high-quality evidence, ensure regulatory compliance, and accelerate timelines. One proven strategy that consistently improves clinical trial outcomes is outsourcing medical and scientific affairs services to experienced partners.
By leveraging specialized expertise, organizations can enhance scientific credibility, optimize clinical strategy, and ensure effective communication across the product life cycle.
Understanding Medical and Scientific Affairs in Clinical Research
Medical and scientific affairs play a critical role in bridging clinical development, regulatory strategy, and scientific communication. These teams are responsible for:
-
Scientific data interpretation
-
Evidence generation and dissemination
-
Support for clinical trial design and execution
-
Alignment of clinical data with regulatory and publication standards
At Curexbio, expert medical scientific affairs services support sponsors across clinical development phases by ensuring data integrity, scientific accuracy, and regulatory readiness.
Key Reasons Outsourcing Medical and Scientific Affairs Improves Trial Outcomes
1. Access to Specialized Scientific Expertise
Outsourcing provides immediate access to experienced medical professionals, therapeutic experts, and regulatory-aware scientific teams. These specialists understand evolving global guidelines and therapeutic nuances, reducing scientific and compliance risks.
This expertise ensures:
-
Robust protocol development
-
Accurate data interpretation
-
Strong alignment with regulatory expectations
2. Improved Clinical Trial Strategy and Design
Early involvement of outsourced medical and scientific affairs teams strengthens trial design by ensuring endpoints, eligibility criteria, and clinical objectives are scientifically justified and regulator-ready.
This results in:
-
Fewer protocol amendments
-
Reduced delays
-
Improved trial efficiency and data quality
3. Enhanced Regulatory Communication and Documentation
Medical and scientific affairs teams work closely with medical writing services to create high-quality regulatory submission documents. Clear, scientifically sound documentation reduces regulatory queries and approval timelines.
Curexbio supports sponsors with compliant documentation through its dedicated medical writing services, including:
-
Protocols
-
Investigator brochures
-
Clinical Study Reports (CSR)
-
Regulatory submission documents
4. Faster Timelines and Cost Efficiency
Building and maintaining an in-house medical affairs team is costly and time-consuming. Outsourcing allows sponsors to scale resources as needed, reducing overhead while maintaining quality.
Benefits include:
-
Faster study start-up
-
Efficient resource utilization
-
Predictable project timelines
5. Stronger Scientific Publications and Data Dissemination
Medical and scientific affairs teams ensure that clinical trial data is communicated accurately and ethically through publications, congress materials, and scientific responses.
This strengthens:
-
Scientific credibility
-
Stakeholder confidence
-
Product value during and beyond clinical development
6. Better Risk Management and Compliance
Outsourced teams bring deep understanding of global regulatory standards, including GCP, ICH, and local authority expectations. This helps identify potential risks early and ensures compliance throughout the clinical trial lifecycle.
Additionally, supporting documents such as health and safety policy statements and compliance documentation are developed in line with regulatory requirements to ensure inspection readiness.
Why Choose Curexbio for Medical and Scientific Affairs Services?
Curexbio delivers integrated medical and scientific affairs services tailored to sponsors, CROs, and biotech companies. With a strong focus on quality, compliance, and scientific excellence, Curexbio supports:
-
Clinical development strategy
-
Evidence generation and communication
-
Regulatory and medical writing alignment
-
End-to-end clinical trial support
By partnering with Curexbio, sponsors gain a trusted extension of their internal teams—ensuring better clinical trial outcomes and regulatory success.
Final Thoughts
Outsourcing medical and scientific affairs services is no longer just a cost-saving decision—it is a strategic move that directly impacts clinical trial success. From improved study design and regulatory communication to stronger scientific publications, the right partner can significantly enhance outcomes.
If you are looking to strengthen your clinical development programs with expert-led support, outsourcing medical scientific affairs to Curexbio offers a proven, compliant, and results-driven approach.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0