How to Choose the Right CRO in India: A Strategic Guide
Choosing the right Clinical Research Organization (CRO) in India is one of the most critical decisions in the drug development lifecycle. The CRO you partner with directly influences study timelines, regulatory compliance, data quality, cost efficiency, and overall trial success.
With India rapidly emerging as a global clinical research hub, sponsors are presented with an abundance of CRO options. While this creates opportunity, it also makes selection more complex. A reliable partner like CurexBio—a full-service clinical trial CRO in India—offers end-to-end support from study design to execution, backed by strong regulatory and pharmacovigilance expertise.
Table of Contents
-
Why India Is a Prime Destination for Clinical Research
-
Key Factors to Consider When Selecting a CRO in India
-
Why Strategic CRO Partnerships Matter
-
Partnering with CurexBio for Long-Term Clinical Success
Why India Is a Prime Destination for Clinical Research
India has firmly established itself as a preferred destination for global clinical trials due to a combination of scientific, operational, and economic advantages. Partnering with an experienced clinical research organization in India allows sponsors to leverage these strengths effectively.
? Diverse & Treatment-Naïve Patient Population
India’s genetically diverse and largely treatment-naïve population enables faster recruitment and generation of robust, globally applicable clinical data—especially valuable for oncology, rare diseases, and complex therapeutic areas.
? Cost-Effective Clinical Operations
Clinical trials conducted in India offer significant cost efficiencies compared to Western markets, without compromising quality or compliance. Leading CROs like CurexBio adhere strictly to international regulatory and ethical standards.
?⚕️ Highly Skilled Clinical Workforce
India has a vast pool of experienced investigators, clinical research professionals, biostatisticians, and English-speaking clinicians, enabling smooth communication and efficient trial management.
? Advanced Infrastructure & Digital Capabilities
Modern hospitals, accredited research sites, and strong IT infrastructure support advanced data management, electronic data capture (EDC), and remote monitoring models.
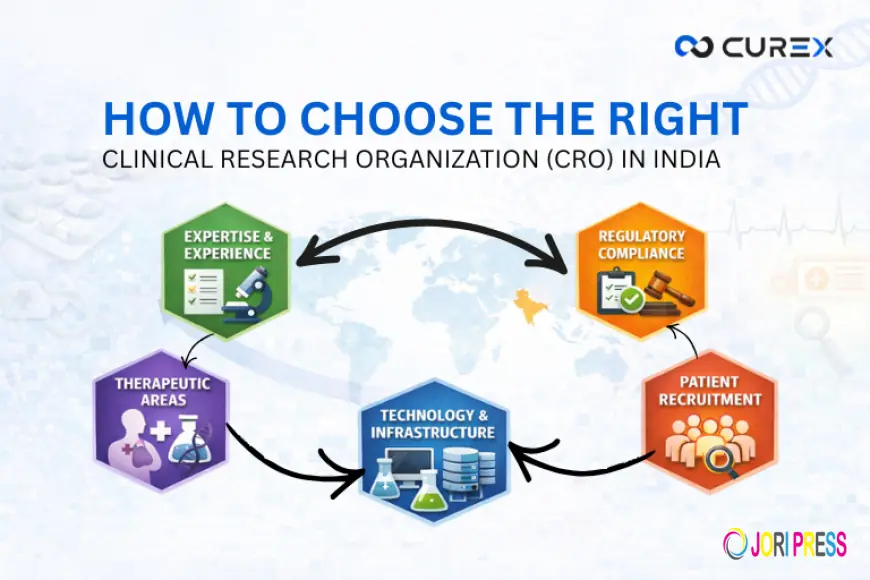
Key Factors for Selecting the Best CRO in India
Not all CROs deliver the same level of expertise or reliability. Evaluating the following critical factors will help sponsors identify the best CRO in India for their specific program needs.
1. Therapeutic & Regulatory Expertise
A strong CRO must demonstrate in-depth therapeutic knowledge and regulatory expertise across global markets.
✔️ What to Look For:
-
Experience across Phases I–IV
-
Expertise in complex therapeutic areas
-
Regulatory navigation with DCGI, USFDA, EMA
-
Strong ICH-GCP compliance
✔️ How CurexBio Aligns:
CurexBio specializes in end-to-end clinical development, ensuring protocols and operations meet both local and global regulatory requirements with precision.
2. Quality Management & Data Integrity
Data quality is the foundation of regulatory approval and scientific credibility.
✔️ What to Look For:
-
Validated Clinical Data Management Systems (CDMS)
-
Compliance with 21 CFR Part 11 and CDISC
-
Real-time data cleaning and audit readiness
✔️ How CurexBio Aligns:
CurexBio employs robust data management and biostatistics solutions designed to maintain the highest standards of data accuracy, security, and traceability throughout the trial lifecycle.
3. Operational Excellence & Delivery Mindset
Timelines matter. A CRO’s operational philosophy can make or break a study.
✔️ What to Look For:
-
Proactive risk management
-
Transparent communication
-
Commitment to timelines over volume
✔️ How CurexBio Aligns:
CurexBio prioritizes quality-driven, time-bound execution, treating every trial as a strategic partnership rather than a transactional project.
4. Comprehensive On-Demand CRO Services
Fragmented vendors often lead to delays and misalignment.
✔️ What to Look For:
-
Integrated clinical trial services
-
Pharmacovigilance and safety reporting
-
Medical & scientific writing support
✔️ How CurexBio Aligns:
As a full-service CRO in India, CurexBio provides clinical trial management, pharmacovigilance, regulatory support, medical writing, and data management under one roof.
5. Technology & Innovation Adoption
Modern trials demand smart, patient-centric technologies.
✔️ What to Look For:
-
Advanced EDC systems
-
Risk-based monitoring (RBM)
-
Digital patient engagement tools
✔️ How CurexBio Aligns:
CurexBio integrates advanced digital platforms and intelligent study designs to enhance operational efficiency, patient safety, and sponsor visibility in real time.
Partnering for Success in a Rapidly Evolving Market
India’s clinical research ecosystem is evolving rapidly, driven by stronger regulations, advanced technologies, and growing demand for specialized biotech and genomics research. In this environment, choosing the right CRO is not just about service offerings—it’s about finding a strategic partner who understands local dynamics while meeting global expectations.
CurexBio exemplifies a partner-centric CRO model built on scientific rigor, regulatory excellence, and uncompromising quality standards. Rather than acting as a conventional service provider, CurexBio positions itself as a trusted ally—supporting sponsors from early development through successful market approval.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0