Internal vs External GMP Audits: Which One Do You Need?
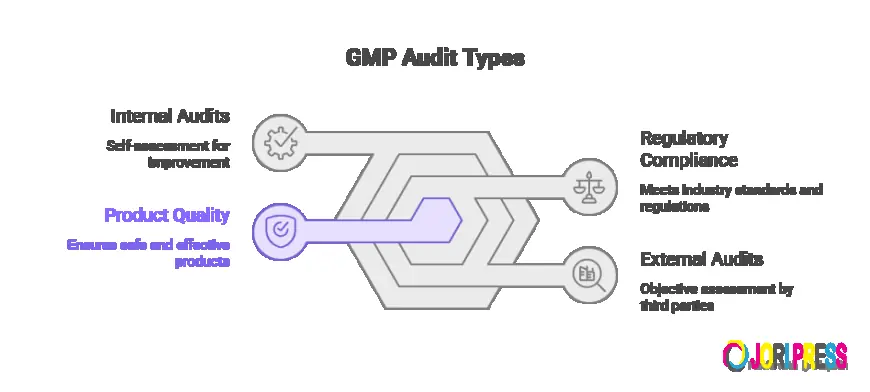
Maintaining Good Manufacturing Practice (GMP) compliance is not optional—it’s a regulatory and business necessity for pharmaceutical, biotech, and API manufacturers. GMP audits play a critical role in ensuring product quality, data integrity, and regulatory readiness.
But one common question companies face is: Should we conduct an internal GMP audit, or engage an external GMP audit company?
The answer depends on your compliance maturity, regulatory exposure, and business objectives.
This article breaks down internal vs external GMP audits, when you need each, and how the right GMP audit consultancy can strengthen your compliance strategy.
What Is a GMP Audit?
A GMP audit is a structured evaluation of manufacturing processes, quality systems, facilities, documentation, and personnel to confirm compliance with global regulatory standards such as US FDA, EMA, MHRA, WHO, and ICH guidelines.
A professional GMP audit services provider evaluates gaps, identifies risks, and recommends corrective and preventive actions (CAPAs) to ensure inspection readiness. Leading organizations rely on experienced partners like a GMP compliance audit company to maintain continuous compliance.
What Is an Internal GMP Audit?
An internal GMP audit is conducted by your own quality assurance team or by an internal GMP audit company operating as an extension of your organization. These audits focus on routine compliance monitoring and process improvement.
Key Objectives of Internal GMP Audits
-
Verify adherence to internal SOPs
-
Identify gaps before regulatory inspections
-
Support annual GMP compliance audit services
-
Build a culture of continuous quality improvement
Advantages of Internal GMP Audits
✔ Familiarity with site operations
✔ Cost-effective for routine monitoring
✔ Supports ongoing compliance programs
✔ Enables proactive CAPA implementation
Limitations
✖ Risk of internal bias
✖ Limited exposure to evolving global regulations
✖ May miss critical inspection-level gaps
Many companies enhance internal audits by engaging a specialized Good Manufacturing Practice audit firm for periodic oversight and benchmarking against industry best practices.
What Is an External GMP Audit?
An external GMP audit is performed by an independent GMP audit service provider with no operational ties to your organization. These audits are typically more rigorous and inspection-oriented.
External audits are often conducted by a pharmaceutical GMP audit company with deep regulatory expertise across multiple regions.
Common Types of External GMP Audits
-
Regulatory readiness audits
-
Vendor and supplier audits
-
Pre-approval inspection (PAI) audits
-
For-cause and risk-based audits
-
GMP audit services for pharmaceutical manufacturers
Advantages of External GMP Audits
✔ Objective and unbiased assessment
✔ Up-to-date regulatory intelligence
✔ High inspection readiness value
✔ Greater credibility with regulators and partners
Organizations frequently engage a cGMP audit company to prepare for FDA, EMA, or WHO inspections or to support global expansion plans.
Internal vs External GMP Audits: Key Differences
| Aspect | Internal GMP Audit | External GMP Audit |
|---|---|---|
| Auditor | Internal QA or internal partner | Independent experts |
| Objectivity | Moderate | High |
| Regulatory Depth | Limited | Extensive |
| Inspection Readiness | Moderate | High |
| Cost | Lower | Higher but value-driven |
| Best For | Routine compliance | Regulatory inspections, due diligence |
When Do You Need an Internal GMP Audit?
You should prioritize internal audits if you:
-
Require routine quality system monitoring
-
Need annual GMP compliance audit services
-
Want early detection of documentation or training gaps
-
Are strengthening internal quality culture
Internal audits are ideal for maintaining baseline compliance but should not replace independent evaluations.
When Do You Need an External GMP Audit?
You should engage an external GMP inspection services provider if you:
-
Are preparing for FDA, EMA, or WHO inspections
-
Need cGMP audit for contract manufacturers
-
Are onboarding new vendors or API suppliers
-
Require vendor GMP audit services for risk mitigation
-
Need objective assessment before regulatory submissions
Many organizations also use remote GMP audit services to assess global facilities efficiently without compromising audit depth.
Supplier and Vendor GMP Audits: A Special Case
Supplier qualification is a major regulatory focus area. Engaging a GMP audit company for API suppliers helps ensure:
-
Raw material quality and traceability
-
Compliance of contract manufacturers
-
Reduced supply chain risk
Specialized GMP supplier audit services are critical for companies relying on global manufacturing and sourcing networks.
Cost Considerations: Internal vs External GMP Audits
While internal audits may appear cost-effective, external audits deliver higher long-term value by preventing costly regulatory findings. Factors influencing GMP audit company pricing/cost include:
-
Scope and complexity of the audit
-
Facility size and product type
-
Regulatory markets involved
-
On-site vs remote audits
Partnering with the best GMP audit company near me often results in faster compliance resolution and reduced inspection risk.
Choosing the Right GMP Audit Partner
An experienced GMP quality audit services provider offers:
-
Global regulatory expertise
-
Risk-based audit methodologies
-
Actionable CAPA recommendations
-
End-to-end compliance support
Zenovel delivers tailored audit solutions as a trusted GMP audit consultancy, supporting pharmaceutical, biotech, and medical device companies across the product lifecycle. Learn more about Zenovel’s integrated quality and compliance solutions.
Final Verdict: Internal, External, or Both?
The most effective compliance strategy is not internal vs external—but internal plus external.
-
Use internal audits for continuous monitoring
-
Use external audits for regulatory readiness and objectivity
This combined approach ensures sustainable compliance, stronger inspection outcomes, and long-term business success.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0