Etorphine Production Process with Cost Analysis
The Etorphine Production Process with Cost Analysis provides an in-depth exploration of the intricate steps involved in producing Etorphine, a powerful opioid used primarily in veterinary medicine, particularly for immobilizing large animals.
Introduction
The Etorphine Production Process with Cost Analysis provides an in-depth exploration of the intricate steps involved in producing Etorphine, a powerful opioid used primarily in veterinary medicine, particularly for immobilizing large animals. As a potent synthetic opioid, Etorphine is a crucial tool in wildlife management and veterinary care, but its production comes with significant regulatory, raw material, and process challenges. This report delves into each aspect of the production process, from procurement resource assessment to the detailed costs involved, enabling businesses to optimize production strategies.
Request For Free Sample:https://www.procurementresource.com/production-cost-report-store/etorphine/request-sample
Procurement Resource Assessment: Etorphine Production Process
Sourcing Raw Materials and Intermediates
Etorphine synthesis requires specialized raw materials and chemical intermediates, sourced globally to maintain production quality and consistency. Procurement involves identifying reliable suppliers for key materials such as:
- Chemical Precursors: The synthesis of Etorphine begins with complex alkaloids, particularly oripavine, a natural opiate alkaloid derived from Papaver bracteatum (Iranian poppy). Sourcing oripavine or its derivatives involves strict regulations due to its controlled status.
- Catalysts and Reagents: Various catalysts and reagents are required to promote chemical reactions during synthesis. Their procurement depends on regional availability and regulatory guidelines concerning chemical handling and use.
Supplier Selection and Logistics
- Reliable Supplier Partnerships: Due to Etorphine's controlled nature, businesses must establish partnerships with highly vetted suppliers. Ensuring regulatory compliance is critical in selecting suppliers of opiate-based intermediates.
- Logistics and Transportation: Special handling and storage protocols are necessary due to the sensitive nature of the materials involved. Secure, licensed logistics partners are required for transporting controlled substances like Etorphine precursors.
Etorphine Overview
Etorphine is a potent opioid that is approximately 1,000 to 3,000 times more powerful than morphine. It was developed in the 1960s and is primarily used in veterinary medicine for the rapid immobilization of large animals, such as elephants and rhinoceroses. Due to its potency, Etorphine is subject to stringent regulations under international narcotics control treaties.
Chemical Properties
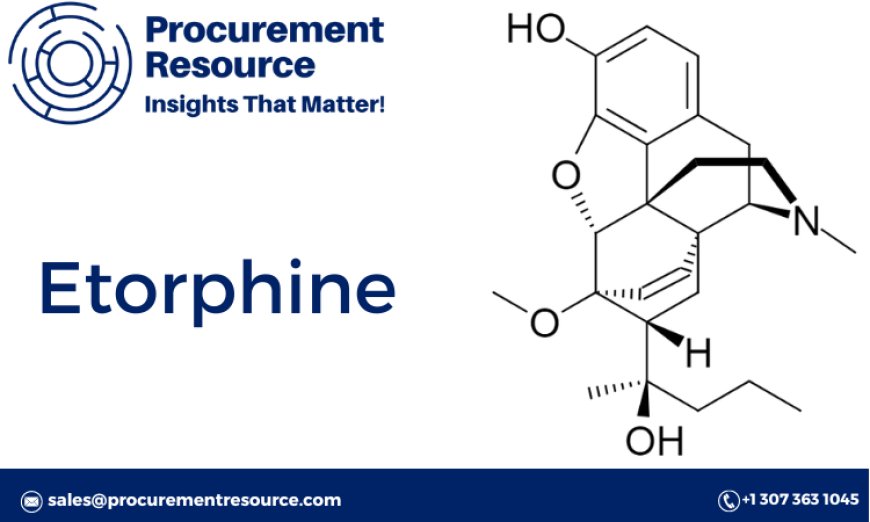
Etorphine, known chemically as (5α,17β)-7,8-dihydro-4,5-epoxy-17-methylmorphinan-6-yl acetate, is a semi-synthetic opioid derived from thebaine, an opiate alkaloid found in poppy plants. Its structure includes an epoxide group and ester functional group, both of which contribute to its high potency and biological activity.
Production Process
The production of Etorphine involves several key steps, beginning with the extraction and isolation of oripavine or thebaine, followed by chemical modifications to produce the final compound:
- Extraction and Isolation: The initial step in Etorphine production is the extraction of oripavine or thebaine from opium poppy derivatives.
- Chemical Synthesis: Chemical modification of the opiate alkaloid to introduce functional groups that enhance Etorphine's potency and efficacy.
- Purification: The product is purified using advanced techniques like crystallization or chromatography to achieve the desired purity levels.
- Formulation: After purification, the Etorphine is formulated into injectable solutions or other forms suitable for veterinary use.
Market Drivers
The global demand for Etorphine is driven by several factors that influence its production and market potential. Key market drivers include:
Veterinary Use in Wildlife Management
Etorphine's primary application is in veterinary medicine, where it is used to immobilize large, wild animals during conservation efforts, medical procedures, or transport. Its potency and fast-acting properties make it ideal for handling animals such as elephants, rhinos, and other megafauna.
Regulatory Environment
Strict regulatory control of Etorphine, both in terms of its production and distribution, plays a significant role in the market. Government agencies like the U.S. Drug Enforcement Administration (DEA) and similar bodies globally control access to Etorphine, limiting its availability to licensed entities.
Growing Conservation Efforts
As wildlife conservation efforts increase globally, the demand for Etorphine as a tool for humane animal management has also risen. National parks, zoos, and wildlife reserves depend on Etorphine to manage animal populations safely, contributing to its sustained demand.
Technological Advancements in Drug Delivery
Technological innovations in the formulation of opioids, including improved delivery systems for injectable anesthetics, are further driving the market for Etorphine. Advanced formulations ensure safe and effective delivery in field conditions.
Raw Materials Requirements
The synthesis of Etorphine requires specific and highly regulated raw materials, most of which fall under strict legal controls. The most critical raw materials include:
Opiate Alkaloids
- Oripavine/Thebaine: These alkaloids are extracted from the opium poppy and serve as the starting material for synthesizing Etorphine. Both are controlled substances, necessitating stringent regulatory compliance in procurement.
Chemical Reagents and Solvents
- Acetic Anhydride: This reagent is used to acetylate certain intermediates during the synthesis process.
- Catalysts: Specific catalysts are employed to facilitate the chemical reactions required for Etorphine production.
- Solvents: High-purity solvents are used in various steps, particularly during the extraction and purification phases.
Excipients
- Formulation Agents: Once synthesized, Etorphine is formulated using excipients that stabilize the active ingredient for veterinary use.
Costs and Key Process Information
The cost structure for producing Etorphine involves several important factors:
Raw Material Costs
The most significant cost factor is the procurement of controlled opiate alkaloids like oripavine and thebaine. Given the strict legal controls and limited availability, these raw materials command high prices. Additionally, sourcing high-purity reagents and solvents adds to production costs.
Manufacturing Costs
- Labor Costs: The synthesis of Etorphine requires skilled chemists and technicians with expertise in handling controlled substances.
- Facility Costs: Specialized facilities that meet regulatory standards for the production of controlled substances are required, which increases overhead costs.
- Energy Consumption: Chemical synthesis processes are energy-intensive, adding to the overall production cost.
Quality Control
Given the potency and regulatory status of Etorphine, stringent quality control measures are essential at every stage of production. Analytical testing, including high-performance liquid chromatography (HPLC) and mass spectrometry, ensures that the final product meets purity and safety standards.
Regulatory Compliance Costs
Compliance with national and international regulations regarding the production and distribution of Etorphine is mandatory. This includes securing licenses, conducting audits, and adhering to strict documentation requirements, all of which contribute to production costs.
Packaging and Distribution
Etorphine is typically formulated as an injectable solution, requiring sterile packaging and secure distribution channels. Packaging costs include sterile vials, syringes, and secure labeling, while distribution requires licensed handlers due to its controlled substance status.
Looking for an Exhaustive and Personalized Report?
For companies involved in Etorphine production or procurement, obtaining an exhaustive and personalized report can be invaluable. Such a report offers:
- Detailed insights into the production process, from raw material procurement to final formulation.
- Cost analysis to help businesses optimize their production strategies.
- Information on regulatory requirements, ensuring compliance in every aspect of Etorphine production.
- Tailored recommendations for improving efficiency and reducing costs.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Benking sley
Email: sales@procurementresource.com
Toll Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537 132103 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA












