Bilastine Production Process with Cost Analysis
The Bilastine Production Process with Cost Analysis is an essential component for understanding the overall efficiency and economic viability of manufacturing this antihistamine medication.
Introduction
The Bilastine Production Process with Cost Analysis is an essential component for understanding the overall efficiency and economic viability of manufacturing this antihistamine medication. Bilastine, a second-generation antihistamine, is primarily used to alleviate symptoms associated with allergic rhinitis and chronic urticaria. This comprehensive report delves into the production process of Bilastine, including an in-depth analysis of procurement resources, raw material requirements, market drivers, and cost factors. By examining these aspects, businesses can gain valuable insights into optimizing production and making informed decisions.
Request For Free Sample:https://www.procurementresource.com/production-cost-report-store/bilastine/request-sample
Procurement Resource Assessment: Bilastine Production Process
The procurement resource assessment for the Bilastine production process involves evaluating various resources essential for efficient manufacturing. This includes assessing the availability and quality of raw materials, supplier reliability, and the logistics involved in resource acquisition.
Raw Materials
The production of Bilastine requires specific raw materials, including active pharmaceutical ingredients (APIs) and excipients. The primary API used is Bilastine, synthesized through a multi-step chemical process. The availability and cost of these raw materials can significantly impact the overall production cost. Reliable suppliers and consistent quality are crucial for maintaining production efficiency and ensuring the efficacy of the final product.
Supplier and Logistics
Assessing potential suppliers for both Bilastine API and excipients involves evaluating their production capabilities, quality control measures, and track record. Logistics play a vital role in ensuring timely delivery of materials and managing inventory levels to avoid production delays.
Bilastine Overview
Bilastine is a potent second-generation antihistamine known for its effectiveness in treating allergic conditions such as hay fever and chronic urticaria. Unlike first-generation antihistamines, Bilastine has a reduced potential for causing drowsiness, making it a preferred choice for patients who need to avoid sedation.
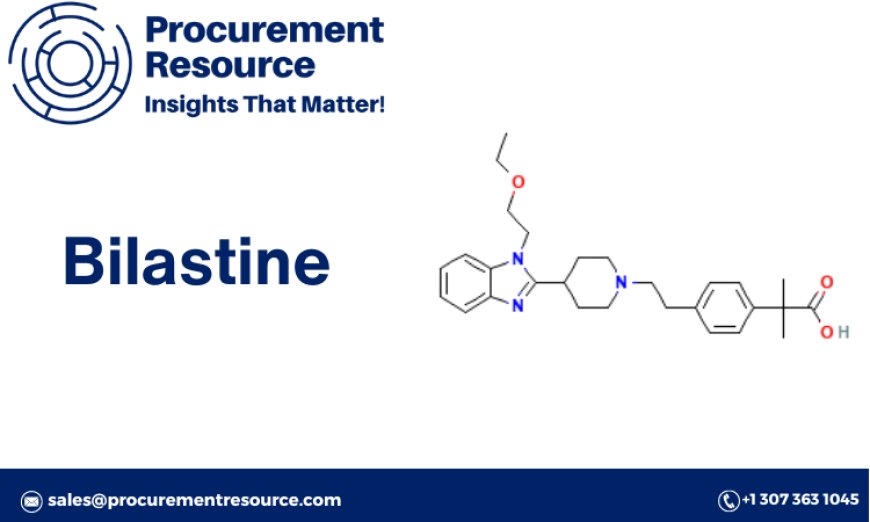
Chemical Properties
Bilastine, chemically known as (2R)-2-[4-(2-(4-(2-hydroxyethoxy)phenyl)piperazin-1-yl)butyl]phenyl]acetic acid, is a selective H1 receptor antagonist. Its chemical structure is designed to provide long-lasting relief from allergy symptoms by blocking histamine receptors without crossing the blood-brain barrier significantly.
Production Process
The production process of Bilastine involves several key steps, including:
- Synthesis of Intermediates: Bilastine is synthesized from various chemical intermediates through a series of reactions.
- Purification: The crude product undergoes purification to remove impurities and achieve the desired purity level.
- Formulation: The purified Bilastine is then formulated into various dosage forms, such as tablets or capsules, ensuring proper dosage and stability.
Market Drivers
Understanding the market drivers for Bilastine is crucial for forecasting demand and optimizing production strategies. Key market drivers include:
Allergy Prevalence
The increasing prevalence of allergic conditions, including seasonal allergies and chronic urticaria, drives the demand for effective antihistamines like Bilastine. Rising awareness and diagnosis of allergic conditions also contribute to market growth.
Regulatory Environment
Regulatory approvals and guidelines for antihistamine medications impact the production and commercialization of Bilastine. Compliance with regulatory requirements ensures the product's safety and efficacy, influencing market acceptance and growth.
Consumer Preferences
Consumer preferences for non-drowsy antihistamines have contributed to the popularity of Bilastine. As more individuals seek effective treatments without sedation, the demand for Bilastine and similar products is expected to rise.
Raw Material Requirements
The production of Bilastine requires specific raw materials, including:
Active Pharmaceutical Ingredients (APIs)
- Bilastine: The primary API, which undergoes chemical synthesis from precursors.
- Intermediates: Chemical intermediates used in the synthesis of Bilastine.
Excipients
- Binders: Used to hold the tablet or capsule together.
- Fillers: To provide the necessary volume and consistency.
- Stabilizers: To enhance the shelf-life and stability of the final product.
Packaging Materials
- Blisters: For tablet or capsule packaging.
- Bottles: For bulk packaging of dosage forms.
Costs and Key Process Information
The cost of producing Bilastine involves several factors:
Raw Material Costs
The cost of raw materials, including Bilastine API and excipients, constitutes a significant portion of the production cost. Fluctuations in raw material prices can impact the overall cost structure.
Manufacturing Costs
Manufacturing costs include expenses related to labor, equipment, utilities, and facility maintenance. Efficient manufacturing processes and technology can help reduce these costs.
Quality Control
Quality control processes ensure that the final product meets regulatory standards and quality specifications. This includes testing for purity, potency, and stability, which contributes to the overall production cost.
Packaging and Distribution
Packaging costs involve materials and processes for preparing the final product for distribution. Distribution costs include logistics, warehousing, and transportation.
Regulatory Compliance
Compliance with regulatory requirements involves additional costs related to documentation, testing, and approvals. These costs ensure that the product meets safety and efficacy standards.
Looking for an Exhaustive and Personalized Report
For businesses involved in the production or procurement of Bilastine, having an exhaustive and personalized report is invaluable. Such a report provides detailed insights into the production process, cost factors, and market dynamics, helping businesses make informed decisions and optimize their operations.
An exhaustive report on Bilastine production not only includes a thorough analysis of procurement resources, raw material requirements, and market drivers but also offers personalized recommendations based on specific business needs and market conditions. This level of detail can significantly substantiate business strategies, enhance operational efficiency, and improve cost management.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Benking sley
Email: sales@procurementresource.com
Toll Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537 132103 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA












