Atrial Fibrillation Device Market Future Shaped by Artificial Intelligence Enabled Cardiac Diagnostic Technologies Adoption.
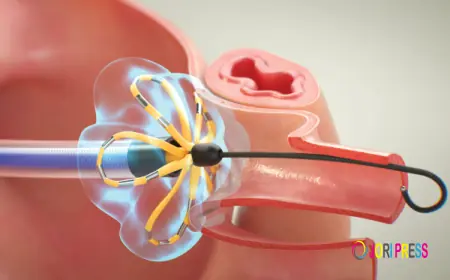
MedLumics has secured an additional €18 million (approximately US $22 million) to support clinical trials of its optical imaging system designed to assist in a surgical procedure for atrial fibrillation (AF) a critical heart condition characterized by an irregular heartbeat.
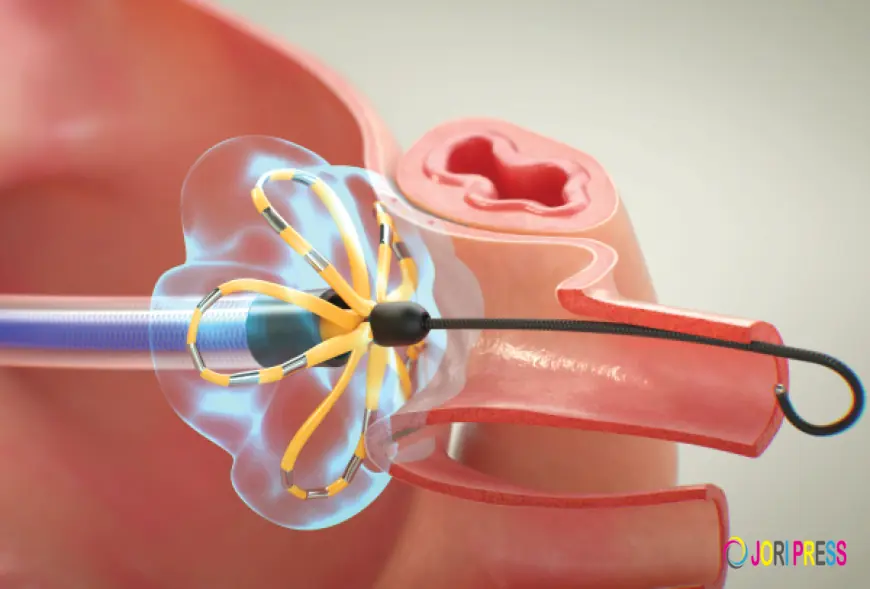
Despite strong growth prospects, the atrial fibrillation (AF) device market faces several significant inhibitors that can slow adoption, restrict accessibility, and challenge long-term expansion. Atrial fibrillation, characterized by irregular and often rapid heart rhythm, requires complex management strategies that frequently involve advanced diagnostic and therapeutic devices. However, economic constraints, clinical limitations, regulatory hurdles, and systemic healthcare challenges collectively influence the pace at which these technologies are deployed worldwide.
One of the most prominent inhibitors is the high cost associated with AF devices and related procedures. Advanced technologies such as catheter ablation systems, implantable cardiac monitors, and left atrial appendage closure devices involve substantial manufacturing, infrastructure, and operational expenses. Hospitals must invest in specialized electrophysiology laboratories, imaging equipment, and trained personnel to perform procedures safely. For patients, out-of-pocket expenses can be considerable, particularly in regions where insurance coverage is limited. These financial barriers can deter both healthcare providers and patients from pursuing device-based treatment options, especially in low- and middle-income countries.
Limited reimbursement policies further compound cost-related challenges. In many healthcare systems, reimbursement frameworks lag behind technological innovation, resulting in partial or inconsistent coverage for advanced procedures. When reimbursement rates fail to reflect the true cost of device implantation and follow-up care, hospitals may hesitate to offer these services. This issue is particularly relevant for newer technologies that lack long-term cost-effectiveness data, making payers cautious about approving widespread coverage.
Another significant inhibitor is the requirement for specialized expertise. Many AF device procedures, particularly catheter ablation, are technically complex and demand highly trained electrophysiologists. The availability of such specialists varies widely across regions, with shortages common outside major urban centers. Training programs require substantial time and resources, and maintaining procedural proficiency often depends on performing a sufficient volume of cases. In areas where expertise is scarce, patients may need to travel long distances to access treatment, reducing overall utilization.
Clinical risks and procedural complications also influence adoption rates. Although device-based therapies have improved considerably, no intervention is entirely risk-free. Potential complications from ablation procedures include bleeding, cardiac perforation, stroke, and damage to adjacent structures. Implantable devices may carry risks of infection, device migration, or malfunction. Even when complication rates are low, the possibility of adverse outcomes can make physicians and patients cautious, particularly when alternative medical therapies are available.
Another important limitation is variable clinical effectiveness across patient populations. Atrial fibrillation is a heterogeneous condition with diverse underlying causes, disease durations, and anatomical variations. While some patients experience long-term relief after a single intervention, others may require repeat procedures or ongoing management. Persistent or longstanding AF can be especially difficult to treat successfully. This variability can lead to uncertainty regarding expected outcomes, influencing decision-making by clinicians, patients, and payers.
Regulatory challenges represent another barrier to market growth. Medical devices must undergo rigorous evaluation to ensure safety and efficacy before receiving approval for clinical use. While these standards are essential for patient protection, they can prolong development timelines and increase costs for manufacturers. Differences in regulatory requirements across countries further complicate global commercialization, requiring separate approval processes and compliance strategies for each region. Delays in approval can limit the availability of innovative technologies in certain markets.
Healthcare infrastructure constraints also play a critical role. Advanced AF treatments require not only specialized equipment but also integrated care systems capable of managing pre-procedural evaluation, intraoperative support, and long-term follow-up. In many parts of the world, hospitals lack the necessary facilities or resources to implement comprehensive arrhythmia care programs. Even where infrastructure exists, limited capacity can lead to long waiting times, discouraging patients from seeking treatment.
Patient-related factors can also inhibit market expansion. Some individuals are reluctant to undergo invasive procedures, particularly older adults or those with multiple comorbidities. Fear of complications, misconceptions about device implantation, and preference for conservative management may reduce acceptance of interventional therapies. Additionally, adherence to post-procedural follow-up and lifestyle modifications is essential for optimal outcomes, yet not all patients are able or willing to maintain these requirements.
Competition from pharmacological treatments presents another challenge. Although medications may not provide definitive solutions for all patients, they remain widely used due to lower upfront costs and non-invasive administration. Physicians may initially favor drug therapy, reserving device-based interventions for cases where medical management fails. This treatment hierarchy can delay or reduce the number of patients receiving advanced procedures.
Data privacy and cybersecurity concerns are emerging inhibitors as well, particularly for devices that rely on wireless connectivity and remote monitoring. Patients and healthcare providers may worry about the security of sensitive medical information transmitted through digital platforms. Regulatory requirements related to data protection can also increase development complexity for manufacturers, potentially slowing innovation.
Finally, disparities in healthcare access contribute to uneven market growth. Rural populations, underserved communities, and regions with limited healthcare funding often face significant obstacles in obtaining advanced cardiac care. Socioeconomic factors, lack of awareness, and insufficient screening programs can result in underdiagnosis of atrial fibrillation, reducing the pool of patients eligible for device-based interventions.
In conclusion, while the atrial fibrillation device market holds substantial promise, its expansion is tempered by a range of inhibitors spanning economic, clinical, regulatory, and systemic domains. High costs, limited reimbursement, specialist shortages, procedural risks, infrastructure gaps, and patient hesitancy all play a role in shaping adoption patterns. Addressing these challenges will require coordinated efforts from policymakers, healthcare providers, manufacturers, and researchers. Innovations that reduce costs, simplify procedures, expand training opportunities, and improve patient education are likely to be key to overcoming these barriers and unlocking the full potential of device-based solutions for atrial fibrillation management.
Tags:
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0