Exploring Fumaric Acid: A Versatile Organic Acid
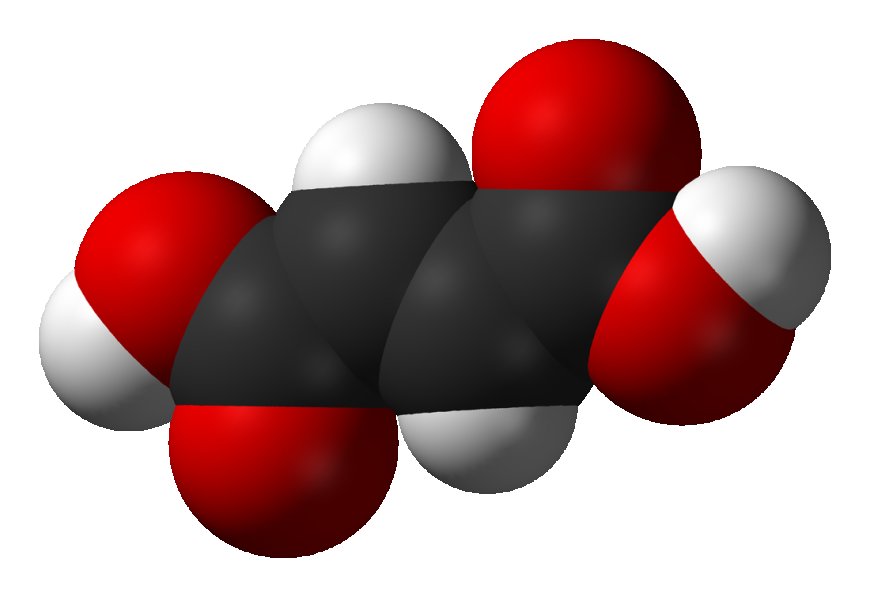
Fumaric acid is an organic compound that is an unsaturated dicarboxylic acid. It has the chemical formula HO2CCH=CHCO2H and exists as a white crystalline powder.
What is Fumaric Acid?
Fumaric acid is an organic compound that is an unsaturated dicarboxylic acid. It has the chemical formula HO2CCH=CHCO2H and exists as a white crystalline powder. Its structure consists of two carboxyl groups connected by an ethylene bridge, giving it two carbon-carbon double bonds.
Uses
It has a variety of industrial and commercial uses due to its ability to act as both an acid and base. Some of its main applications include:
Food Additive
As a food additive, it is approved for use as an acidity regulator in beverages and baked goods. It serves as a flavor enhancer and preservative while also extending the shelf life of products. It is often used in soft drinks, candy, preserves, gelatin desserts, and other processed foods where it contributes a slightly tart taste.
Unsaturated Polyester Resins
It is commonly employed as a reactive plasticizer and crosslinking agent in the production of unsaturated polyester resins (UPRs). UPRs are thermosetting polymers that harden when exposed to heat. They find use in making fiberglass boats, automotive parts, and home construction materials due to their strength and durability. Fumaric Acid improves the mechanical and chemical properties of the final resin products.
Pharmaceuticals
Due to its anti-inflammatory and immunomodulating effects, its derivatives have applications in the pharmaceutical industry. Some conjugate fumarates approved for medical use include:
- Dimethyl fumarate (Tecfidera), prescribed for the treatment of multiple sclerosis by suppressing immune cells in the central nervous system.
- Budesonide fumarate, an oral corticosteroid used to treat Crohn's disease and ulcerative colitis.
- Diphenyl fumarate, an experimental drug in clinical trials for treatment of cancer and autoimmune disorders.
Production Methods
It can be synthesized by various chemical processes involving maleic acid as the starting material. The main production methods are:
- Catalytic hydration of maleic anhydride - Maleic anhydride reacts with water in the presence of an acid catalyst to yield fumaric acid. This is currently the most common industrial process.
- Oxidation of butane - Butane is oxidized to produce maleic anhydride, which is then hydrated to fumaric acid.
- Fermentation - Certain fungi like Rhizopus species can ferment sugars to produce it. This "green" biotechnological route is gaining interest as a sustainable alternative.
Properties
It is a white crystalline solid with the following physical and chemical properties:
- Molecular formula: C4H4O4
- Molecular weight: 116.07 g/mol
- Melting point: 287-289°C
- Solubility: Very soluble in water, methanol, ethanol; slightly soluble in chloroform
- Acidity: Strong acid with pKa of 3.03
- Structure: Cis-trans isomerism due to double bonds; trans isomer predominates
- Polymorphism: 2 crystal forms - normal and monoclinic
- Stability: Thermally stable up to 300°C; light sensitive
The acidic properties of its two carboxyl groups allow it to act as a diacid under neutral or basic conditions. The double bond system imparts cis-trans isomerism and conjugation of electrons over the molecule. These unique structural aspects contribute to its reactivity and applications profile.
Uses of Derivatives
Functionalization of fumaric acid through esterification or other reactions yields a range of valuable fumarate derivatives with various applications:
- Dibutyl fumarate - Plasticizer for PVC, unsaturated polyester resins and alkyd resins
- Diethyl fumarate - Intermediate for pharmaceutical and agrochemical syntheses
- Dimethyl fumarate - Oral treatment for multiple sclerosis as mentioned before
- Calcium, copper and zinc fumarates - Feed supplements for livestock, poultry and aquaculture
- Fumaric acid diesters - Green solvents and platform chemicals for various sectors
It is a multifunctional platform chemical due to the presence of acidic carboxyl groups and unsaturated carbon-carbon double bonds in its structure. It undergoes crosslinking, esterification, hydration and other reactions. This enables its widespread usage as a food additive, resin crosslinker, pharmaceutical intermediate and precursor for tailored derivatives. With green production methods also emerging, fumaric acid will likely maintain its relevance as a building block in speciality chemicals and materials industries.
Get more insights on Fumaric Acid
For Deeper Insights, Find the Report in the Language that You want
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc.












