Navigating the Regulatory Landscape: Your Gateway to the Brazilian Market
DDReg specializes in navigating Brazil's complex regulatory landscape, offering expert guidance to ensure full compliance with ANVISA standards. They assist manufacturers of pharmaceuticals, medical devices, cosmetics, and food supplements with end-to-end product registration and market access. Their comprehensive services range from initial dossier preparation and gap analysis to in-country representation and lifecycle management. By combining global experience with local insights, they help companies secure accelerated approvals and maintain sustained compliance in the Brazilian market.

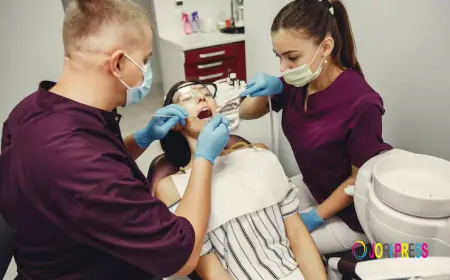
Entering the Brazilian market requires navigating the complex standards set by ANVISA (Agência Nacional de Vigilância Sanitária), the national regulatory agency responsible for overseeing health-related products. Whether you are a global manufacturer or a niche developer, understanding local compliance is crucial for commercial success. Partnering with experts who specialize in Regulatory Affairs Services in Brazil ensures that your product journey—from dossier preparation and gap analysis to final approval—is smooth, efficient, and fully aligned with national safety and quality mandates.
For companies in the life sciences sector, the requirements for drug approval are particularly rigorous and constantly evolving. Expert guidance is essential for managing Pharmaceutical Regulatory Affairs in Brazil, covering everything from initial marketing authorization to lifecycle maintenance. Similarly, the growing demand for advanced therapies makes Biologics Product Registration in Brazil a critical step for innovators looking to introduce vaccines, monoclonal antibodies, and other biological products to this vast Latin American demographic.
Beyond pharmaceuticals, the healthcare and beauty industries are seeing significant growth, requiring manufacturers to adhere to specific technical standards for market access. Utilizing professional Brazil Medical Device Registration Services can help navigate the intricate classification, certification, and GMP inspection processes required for medical equipment. Concurrently, the personal care market is booming, and streamlined Cosmetic Product Registration in Brazil ensures your beauty and hygiene brands reach consumers without unnecessary regulatory delays.
Finally, as consumer health awareness rises, so does the demand for wellness and nutritional products. Navigating the specific rules for Food Supplements Registration in Brazil requires a deep understanding of permissible ingredients, safety limits, and labeling claims. By leveraging end-to-end regulatory support, companies can ensure all their product categories, including nutraceuticals, meet ANVISA’s stringent expectations, securing a strong foothold in one of the world's most dynamic economies.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0