In Vitro Diagnostics Quality Control Market Emerging Trends and Opportunities through 2030
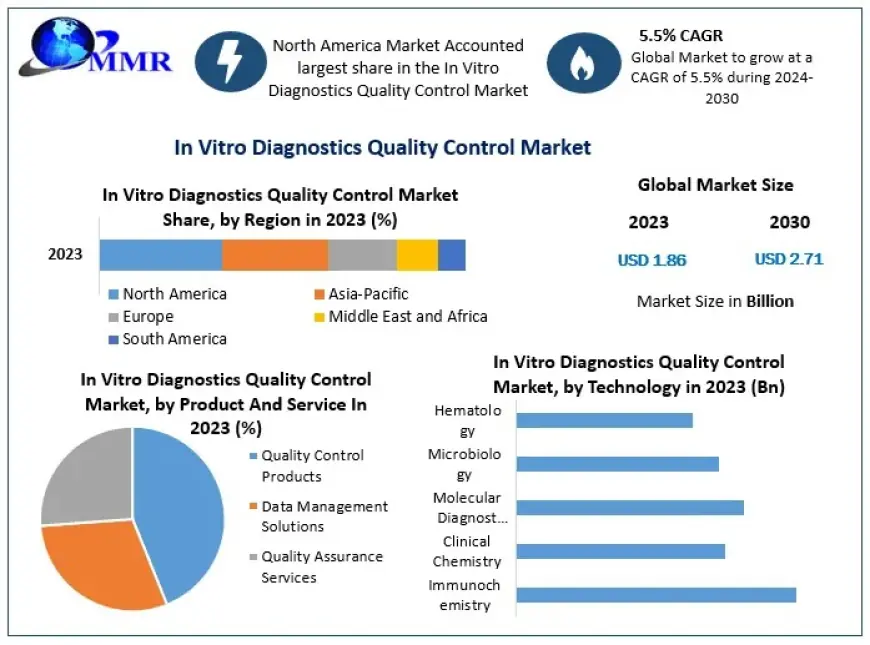
The In Vitro Diagnostics Quality Control Market size was valued at USD 1.86 Bn. in 2023 and the market is expected to grow at 5.5% from 2024 to 2030, reaching nearly USD 2.71 Bn.
In Vitro Diagnostics Quality Control Market Overview: Key Insights and Analysis
Maximize Market Research, a leading business consultancy firm, has released an in-depth report on the “In Vitro Diagnostics Quality Control Market,” offering comprehensive insights into market dynamics, demand trends, pricing strategies, and the competitive landscape. This detailed analysis sheds light on the current state of the In Vitro Diagnostics Quality Control Market and delivers accurate forecasts extending through 2030, equipping businesses with the knowledge needed to navigate and capitalize on emerging opportunities.
Download Your Full PDF Sample of the In Vitro Diagnostics Quality Control Market Report Now: https://www.maximizemarketresearch.com/request-sample/161874/
In Vitro Diagnostics Quality Control Market Scope and Methodology: A Detailed Approach
The study incorporates both descriptive analysis and SWOT analysis as integral components of the research process. Aimed at providing detailed market insights on the In Vitro Diagnostics Quality Control Market topic, the research utilizes data collection methods such as surveys and questionnaires. Once gathered, the data undergoes rigorous analysis using mathematical, statistical, and numerical approaches. By leveraging both qualitative and quantitative research techniques, this study ensures accurate tracking of market trends and developments.
The market is analyzed comprehensively, focusing on future growth potential, innovative R&D efforts, industry-specific practices, strategic development initiatives, and consolidation activities, including mergers and acquisitions. Organizational strategies, leadership portfolios, and frameworks of prominent global CEOs are also examined. Additionally, the report employs tools like SWOT and PESTLE analysis to evaluate microeconomic factors and uncover critical market trends, ensuring actionable insights for stakeholders.
Understanding Market Dynamics: What Drives the In Vitro Diagnostics Quality Control Market?
It costs a lot of money to install a QC process in a clinical laboratory. Additionally, laboratories need to have dedicated staff to manage the QC system. Additionally, the costs of QC procedures are the same regardless of the number of tests that are finished. As a result, clinical laboratories that conduct small numbers of diagnostic tests find the cost of establishing QC procedures to be unaffordable. This is anticipated to lead to a decline in the market for in vitro diagnostics quality control, as well as financial constraints in many hospitals and labs in both developed and developing nations. Additionally, the cost of QA services for independent diagnostic test evaluation and data management solutions to handle QC data increase the overall cost for end users.
In Vitro Diagnostics Quality Control Market Segmentation: Unveiling Insights by Module
by Product And Service
1. Quality Control Products
2. Data Management Solutions
3. Quality Assurance Services
by Technology
1. Immunochemistry
2. Clinical Chemistry
3. Molecular Diagnostics
4. Microbiology
5. Hematology
6. Coagulation/ Hemostasis
7. Other Technologies
by End User
1. Hospitals
2. Clinical Laboratories
3. Academic and Research Institutes
4. Home-care
5. Other End users
by Manufacturer Type
1. IVD Instrument Manufacturers
2. Third Party Quality Control Manufacturers
2.1. Independent third party quality controls
2.2. Instrument specific third party quality controls
Curious to see what’s inside? Get your sample copy of the report today
Leading Global Manufacturers: Who Dominates the In Vitro Diagnostics Quality Control Market?
1. Bio-Rad Laboratories, Inc.
2. Randox Laboratories Ltd.
3. Thermo Fisher Scientific, Inc.
4. LGC Limited
5. Abbott Laboratories
6. Roche Diagnostics
7. Siemens Healthineers
8. Danaher Corporation
9. Fortress Diagnostics
10. SERO AS
11. Sysmex Corporation
12. Ortho-Clinical Diagnostics
13. Helena Laboratories Corporation
14. Quidel Corporation
15. Sun Diagnostics, LLC.
16. Seegene Inc.
17. ZeptoMetrix Corporation
18. Qnostics
19. Bio-Techne Corporation
20. Microbiologics
21. Microbix Biosystems
22. Streck, Inc.
23. Alpha-Tec Systems
24. Maine Molecular Quality Controls, Inc.
25. Grifols, S.A.
Regional Market Progress: How Are Different Regions Performing in the In Vitro Diagnostics Quality Control Market?
Evaluating the market's potential involves analyzing various critical factors such as buyer-direct agreements, innovative research and development activities, strategic business models, and significant mergers and acquisitions. The study also incorporates detailed organizational structures, corporate goals, executive portfolios, and an in-depth assessment of top industry leaders. Through a thorough application of SWOT and PESTLE analysis, the research identifies key microeconomic influences and emerging market trends, offering a well-rounded perspective on growth opportunities and challenges.
Key questions answered in the In Vitro Diagnostics Quality Control Market are:
- What is the current size and scope of the In Vitro Diagnostics Quality Control Market?
- What is the growth rate projected for the In Vitro Diagnostics Quality Control Market?
- What was the market size of In Vitro Diagnostics Quality Control Market in the last fiscal year?
- What upcoming opportunities and trends are shaping the In Vitro Diagnostics Quality Control Market?
- What are the different segments within the In Vitro Diagnostics Quality Control Market?
- What recent industry trends can be leveraged to generate additional revenue streams for the In Vitro Diagnostics Quality Control Market?
- Which segments are covered in the In Vitro Diagnostics Quality Control Market analysis?
- What are the key factors expected to drive the growth of the In Vitro Diagnostics Quality Control Market?
- What growth strategies are companies considering to enhance their presence in the In Vitro Diagnostics Quality Control Market?
- Who are the leading players in the In Vitro Diagnostics Quality Control Market, and what are their key offerings?
- Who are the major players impacting the In Vitro Diagnostics Quality Control Market?
- What is the projected Compound Annual Growth Rate (CAGR) for the In Vitro Diagnostics Quality Control Market during the forecast period?
For additional reports on related topics, visit our website:
Warehouse Automation System Market https://www.maximizemarketresearch.com/market-report/warehouse-automation-system-market/167432/
global Lithium-Ion Battery Recycling Market https://www.maximizemarketresearch.com/market-report/lithium-ion-battery-recycling-market/524/
About Us:
Maximize Market Research is one of the fastest-growing market research and business consulting firms serving clients globally. Our revenue impact and focused growth-driven research initiatives make us a proud partner of majority of the Fortune 500 companies. We have a diversified portfolio and serve a variety of industries such as IT & telecom, chemical, food & beverage, aerospace & defense, healthcare and others.
Contact Maximize Market Research:
MAXIMIZE MARKET RESEARCH PVT. LTD.
⮝ 3rd Floor, Navale IT park Phase 2,
Pune Banglore Highway, Narhe
Pune, Maharashtra 411041, India.
✆ +91 9607365656
???? sales@maximizemarketresearch.com
www.maximizemarketresearch.com












