Anacetrapib Production Process with Cost Analysis
The production of Anacetrapib, a potent cholesterol-lowering drug, requires a detailed understanding of its manufacturing process, resource allocation, and cost implications
Introduction
The production of Anacetrapib, a potent cholesterol-lowering drug, requires a detailed understanding of its manufacturing process, resource allocation, and cost implications. This report delves into the Anacetrapib production process, offering an in-depth cost analysis and exploring the key factors that influence its production. For businesses seeking to optimize their pharmaceutical production processes, this report provides invaluable insights into procurement strategies, raw material requirements, market drivers, and essential cost-related information.
Request For Free Sample: https://www.procurementresource.com/production-cost-report-store/anacetrapib/request-sample
Procurement Resource Assessment: Anacetrapib Production Process
Effective procurement strategies are crucial for ensuring a smooth and cost-efficient production process for Anacetrapib. The procurement resource assessment involves identifying and securing high-quality raw materials, negotiating with suppliers, and ensuring timely delivery to maintain production schedules. This process starts with evaluating potential suppliers based on their reliability, quality standards, and pricing. A thorough understanding of global supply chains is necessary to mitigate risks related to supply disruptions, price fluctuations, and regulatory changes.
In the Anacetrapib production process, the procurement phase is particularly important due to the complexity of the chemical synthesis involved. The production of Anacetrapib requires specific chemicals and intermediates that must meet stringent quality standards. The procurement team must work closely with R&D and quality control departments to ensure that all materials comply with the necessary specifications, thus preventing any impact on the final product’s efficacy and safety.
Understanding Anacetrapib: The Production Process
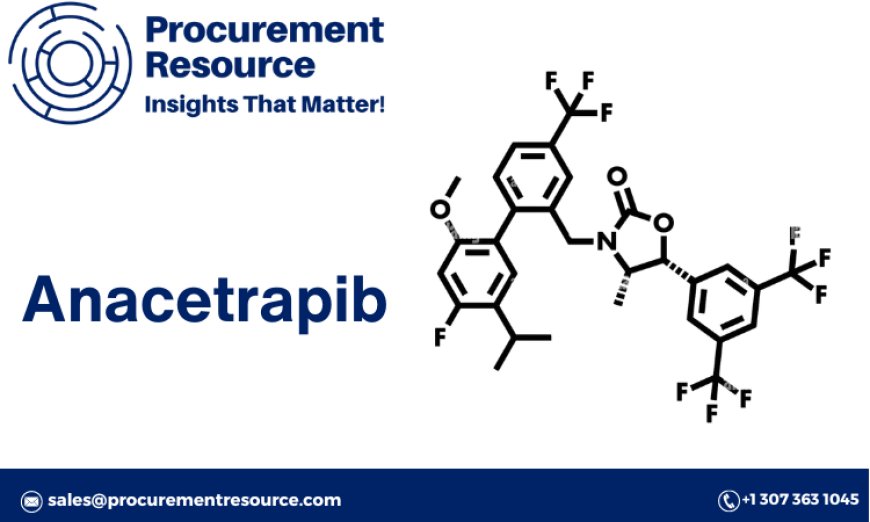
Anacetrapib is a cholesterol ester transfer protein (CETP) inhibitor, which helps reduce low-density lipoprotein (LDL) cholesterol levels and increase high-density lipoprotein (HDL) cholesterol levels in the blood. The production process of Anacetrapib involves multiple stages, including chemical synthesis, purification, and formulation.
The synthesis of Anacetrapib starts with the preparation of intermediate compounds through a series of chemical reactions. These intermediates undergo further transformations to form the active pharmaceutical ingredient (API). The synthesis process requires precise control of reaction conditions, including temperature, pressure, and pH, to ensure the purity and yield of the final product.
After synthesis, the API undergoes purification processes such as crystallization and filtration to remove impurities. The purified API is then formulated into the final dosage form, which may involve mixing with excipients, granulation, and tablet pressing. Each step in the production process is carefully monitored and controlled to meet the required quality standards and regulatory requirements.
Market Drivers: Factors Influencing Anacetrapib Production
The production of Anacetrapib is influenced by several market drivers that affect both demand and supply. Understanding these drivers is essential for businesses to remain competitive and adapt to changing market conditions.
-
Rising Prevalence of Cardiovascular Diseases: The increasing incidence of cardiovascular diseases globally is a significant driver for the demand for cholesterol-lowering drugs like Anacetrapib. As healthcare providers seek effective treatments to manage cholesterol levels in patients, the demand for Anacetrapib is expected to grow.
-
Advancements in Pharmaceutical Manufacturing: Innovations in pharmaceutical manufacturing processes have made it possible to produce drugs like Anacetrapib more efficiently and at a lower cost. Continuous improvements in chemical synthesis, automation, and quality control contribute to the scalability and profitability of Anacetrapib production.
-
Regulatory Environment: The pharmaceutical industry is highly regulated, and changes in regulations can impact the production and distribution of Anacetrapib. Compliance with regulatory standards is essential for market access, and any changes in regulations may require adjustments to the production process.
-
Competition and Patent Expirations: The market for cholesterol-lowering drugs is competitive, with multiple players offering similar products. Patent expirations can lead to increased competition from generic manufacturers, affecting the pricing and profitability of Anacetrapib. Companies must be proactive in managing their intellectual property and exploring opportunities for product differentiation.
Raw Materials Requirements for Anacetrapib Production
The production of Anacetrapib relies on specific raw materials, including chemical reagents, solvents, and intermediates. The quality and availability of these materials directly impact the efficiency and cost of the production process.
-
Chemical Reagents: The synthesis of Anacetrapib requires a variety of chemical reagents, which must be of high purity to ensure the desired chemical reactions occur. These reagents include organic compounds, catalysts, and acids that facilitate the formation of the API.
-
Solvents: Solvents are used in various stages of the production process, including the synthesis and purification of the API. The selection of appropriate solvents is critical for optimizing reaction conditions and achieving high yields. Common solvents used in Anacetrapib production include ethanol, methanol, and acetone.
-
Intermediates: Intermediate compounds are synthesized during the production process and serve as building blocks for the final API. These intermediates must be produced with high precision and purity to ensure the quality of the final product.
-
Excipients: Excipients are non-active ingredients that are combined with the API to create the final dosage form. These materials include binders, fillers, and lubricants, which contribute to the stability, bioavailability, and manufacturability of the final product.
Costs and Key Process Information
The cost of producing Anacetrapib is influenced by several factors, including raw material costs, labor, energy consumption, and capital investments in manufacturing equipment. A comprehensive cost analysis is essential for understanding the economic viability of Anacetrapib production and identifying areas for cost optimization.
-
Raw Material Costs: The cost of raw materials represents a significant portion of the total production cost. Fluctuations in the prices of chemical reagents, solvents, and intermediates can impact the overall cost structure. Companies must continuously monitor market prices and explore opportunities for bulk purchasing or long-term supply agreements to manage raw material costs effectively.
-
Labor and Overhead Costs: Labor costs include the wages of production staff, quality control personnel, and R&D teams involved in the Anacetrapib production process. Overhead costs encompass expenses related to facility maintenance, utilities, and administrative support. Companies can optimize these costs by implementing lean manufacturing practices and investing in employee training.
-
Energy Consumption: The production of Anacetrapib requires energy-intensive processes such as heating, cooling, and mixing. The cost of energy consumption can be a significant factor, especially in regions with high energy prices. Investing in energy-efficient equipment and renewable energy sources can help reduce energy costs and improve sustainability.
-
Capital Investments: The production of Anacetrapib requires specialized manufacturing equipment, including reactors, filtration systems, and tablet presses. The initial capital investment in these assets, as well as ongoing maintenance costs, must be factored into the overall cost analysis. Companies should consider the total cost of ownership when making decisions about equipment procurement.
-
Regulatory Compliance Costs: Compliance with regulatory requirements is essential for the production and distribution of Anacetrapib. These costs include expenses related to quality control testing, documentation, and inspections by regulatory authorities. Companies must allocate sufficient resources to ensure compliance and avoid costly delays or penalties.
Looking for an Exhaustive and Personalized Report That Could Significantly Substantiate Your Business?
For businesses looking to optimize their Anacetrapib production process, a comprehensive and personalized report can provide actionable insights. Such a report should include a detailed analysis of procurement strategies, raw material requirements, market drivers, and cost structures. By leveraging this information, companies can make informed decisions that enhance production efficiency, reduce costs, and improve market competitiveness
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Benking sley
Email: sales@procurementresource.com
Toll Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537 132103 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA












